UPDATE: Pfizer's Coronavirus Vaccine Is Now 95 Percent Effective
The drug maker will apply for emergency FDA approval “within days.”
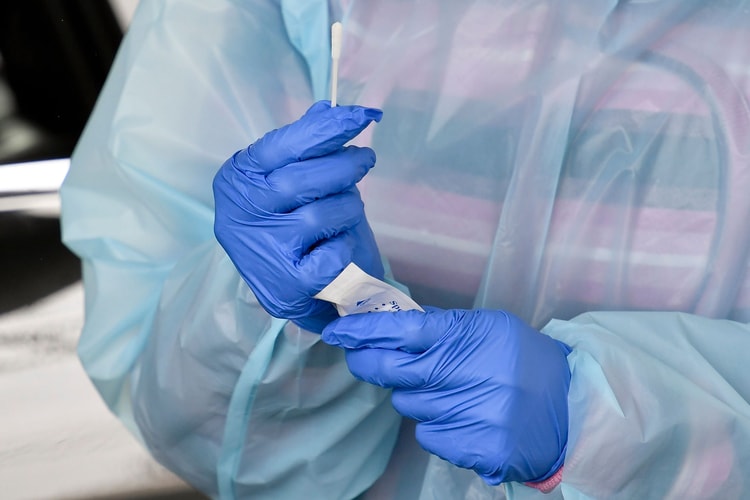
UPDATE (November 18, 2020): Pfizer has announced new data from the late stages of its coronavirus vaccine trial, reporting that the shot is 95 percent effective at preventing both mild and serious cases of the virus and has no serious side effects. According to The New York Times, Pfizer will seek emergency Food and Drug Administration (FDA) approval of the two-dose vaccine “within days.”
If the FDA gives the green light, Pfizer could have up to 50 million doses of the vaccine by the end of 2020 and 1.3 billion by the end of 2021. That being said, only half of those doses — enough for approximately 12.5 million patients — would go to Americans this year. Priority would be given to high-risk groups such as healthcare workers and seniors.
Once the pharmaceutical company has submitted the vaccine for FDA approval, the agency will review its clinical trial and safety data. The FDA will also ask an outside panel of experts to assess the vaccine, a process that could take weeks.
ORIGINAL STORY (November 9, 2020): Biopharmaceutical company Pfizer announced that early data from its coronavirus vaccine trial, reviewed by a panel of independent experts, indicated positive results. According to a report by The New York Times, the anxiously awaited vaccine is more than 90 percent effective at preventing the virus in trial participants with no history of prior COVID-19 infection.
Pfizer announced the promising findings in a press release, rather than in a peer-reviewed medical journal. The 90 percent efficacy rate the company cited could change as trials continue. Pfizer will ask the Food and Drug Administration (FDA) for emergency approval of the vaccine at the end of November, once it has collected two months of safety data. Company executives also told the Times that, by the end of 2020, it will have manufactured enough doses for 15 to 20 million people.
Kathrin Jansen, senior vice president and head of vaccine development at Pfizer, said that an independent board reviewing early data from the clinical trial has not provided company executives with information the FDA will need to authorize the vaccine — including how many trial participants developed mild and severe cases of COVID-19, and how the company will manufacture millions of doses consistently and safely. Still, Jansen remained optimistic: “This is a historical moment,” she said. “This was a devastating situation, a pandemic, and we have embarked on a path and a goal that nobody ever has achieved — to come up with a vaccine within a year.”